Preclinical Safety
Pharmacology Services
Safety Pharmacology is an essential step for drug approval and entry into the global marketplace. These studies take considerable time and money and require specialized capabilities. Delays due to inefficient processes, inaccurate data, or unexpected challenges in the preclinical development process can create lost business opportunities. The choice of models and adherence to regulatory requirements must be carefully strategized for in-vivo studies that leverage safety pharmacology expertise.
AmplifyBio utilizes over 30 years of experience leading preclinical research, creating toxicological solutions, developing modern pharmacokinetics, and assisting with many other extensive studies. We work with our client partners, providing experienced study designs for chosen models (human and animal trials) with physiological expertise to interpret and implement data in real time. Our skilled team can adjust as needed and will conduct testing that meets regulatory requirements, including ICH standards.
Safety Pharmacology Assessment Capabilities
AmplifyBio coordinates and curates customized safety pharmacology studies in a wide variety of animal models. The most common testing applications are cardiovascular, central nervous system (CNS), pulmonary, and renal therapies. Our assessment capabilities include:

- Radiotelemetry
- Plethysmography
- Anesthetized Models
Radiotelemetry of physiological data, both implanted and external (JET) devices
- Electrocardiograms (ECGs) – Heart rate, rhythm, and arrhythmia analysis
- Blood pressures
- Ventricular pressure readings
- Respiratory rate and tidal volume
- Temperature and activity data
- Electroencephalograms (EEGs)
- Species: rat, dog, pig, non-human primates
Plethysmography
- Respiratory rate and tidal volume
- Inspiratory and Expiratory flows and durations
- Indices of pulmonary resistance
- Direct measurement of pulmonary resistance and compliance (when coupled with radiotelemetry for intrathoracic pressure data)
- Real-time pulmonary parameters collected with aerosol delivery of test article
- Species: rat, dog, pig, non-human primates
Anesthetized Models
- Pressure volume loops
- Hemodynamic parameters
- Ultrasound evaluations
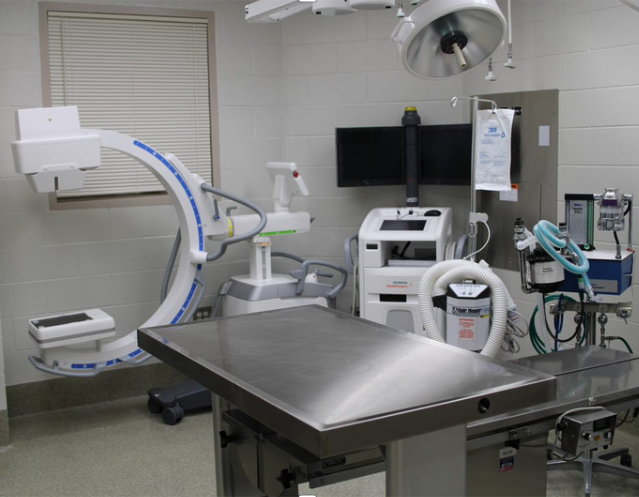
- Cardiac catheterizations using fluoroscopy
- Species: mouse, rat, dog, pig, non-human primates
The AmplifyBio Difference
At AmplifyBio, we use more than safety pharmacology expertise and best practices to assist with testing. We know that picking the best animal model, designing the right study, and being prepared to analyze all data with pharmacology and physiological expertise positions us to expect the unexpected and adjust at a moment’s notice.
- Accurate, reliable results for every study
- Industry-leading safety pharmacology research and study group
- World-class in-house surgical suites with full veterinary staff and surgical support
- ICH, FDA, EPA, GLP, and many other compliance requirements met
- Support for numerous animal types and dose routes
AmplifyBio Preclinical CRO Services
Preclinical CRO services play a crucial role in drug development by conducting studies and experiments on potential therapeutics before they advance to clinical trials. AmplifyBio offers a wide-range of preclinical CRO services including:
AmplifyBio Additional Services
Contact Us
With the expertise to design the right study for every model and flexibility for data-driven implementation, Amplifybio is your partner to trust for in-vivo safety pharmacology assistance. Talk with us and discover how we can support your preclinical studies and compliance requirements.